
PROTOCOL TITLE
Flow cytometry-assisted method for cell disruption of microalgae
AUTHORS
Daniel Figueiredo, Teresa Lopes da Silva, Alice Ferreira, Alberto Reis, Luisa Gouveia
BOOK
Protocolos de microalgas de la Red Renuwal-I
PUBLISHER
CYTED
ISBN
978-84-15413-46-2
ABSTRACT
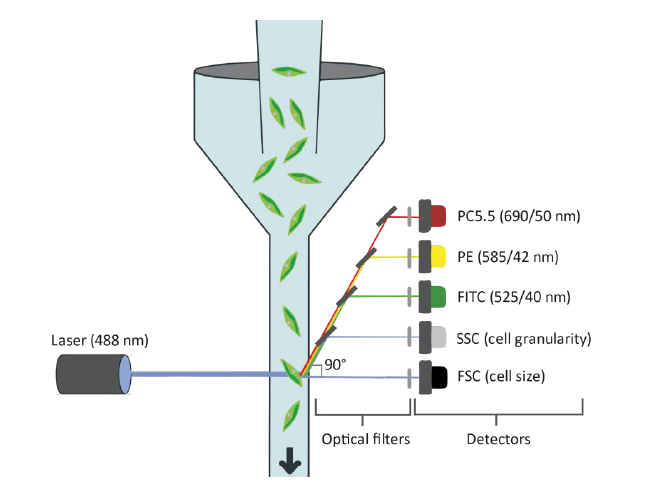
Cell disruption is a mandatory process to extract intracellular components of microalgae, which are useful for food, feed, agriculture, biomedical or pharmaceutical applications. A wide variety of cell disruption technologies is available, but optimization of operation parameters is required. Flow cytometry (FC) combined with viability staining dyes is a valuable tool to quickly analyze cell disruption. In this protocol, samples of Tetradesmus obliquus that are subjected to multiple disruption conditions (e.g., high-pressure homogenization pressures and cycles) are analyzed using a flow cytometer equipped with a 488 nm argon laser. Samples are stained with the viability dye SYTOX-Green, which stains DNA of membrane-compromised cells. Staining optimization is the most critical step in this protocol, since it depends heavily on species, cultivation media and cell concentration. Fluorescence data collected with Forward and Side Scatter detectors as well as the FITC (green) fluorescence detector allows the distinction of intact, membrane-permeabilized and disrupted cell populations. The distinction of cells with compromised membranes can be useful when mild-disruption applications are necessary. In conclusion, by having a faster and more reliable evaluation of cell disruption, rupturing technologies can be optimized quicker, resulting in a more efficient and cost-reduced downstream process.



