
TITLE
Microalgae-associated Stenotrophomonas maltophilia enhances lutein production and biostimulant activity in Monoraphidium sp.
JOURNAL
Algal Research
AUTHORS
João Artur Câmara Manoel, Karolina Sterbová, Mohit Kumar Saini, Daniela Bárcenas-Pérez, José Cheel, Tomás Grivalský, Gergely Ernö Lakatos, Martin Lukes, Petra Urajová, Alice Ferreira, Daniel Figueiredo, Luisa Gouveia, Jirí Masojídek, Kumar Saurav
ABSTRACT
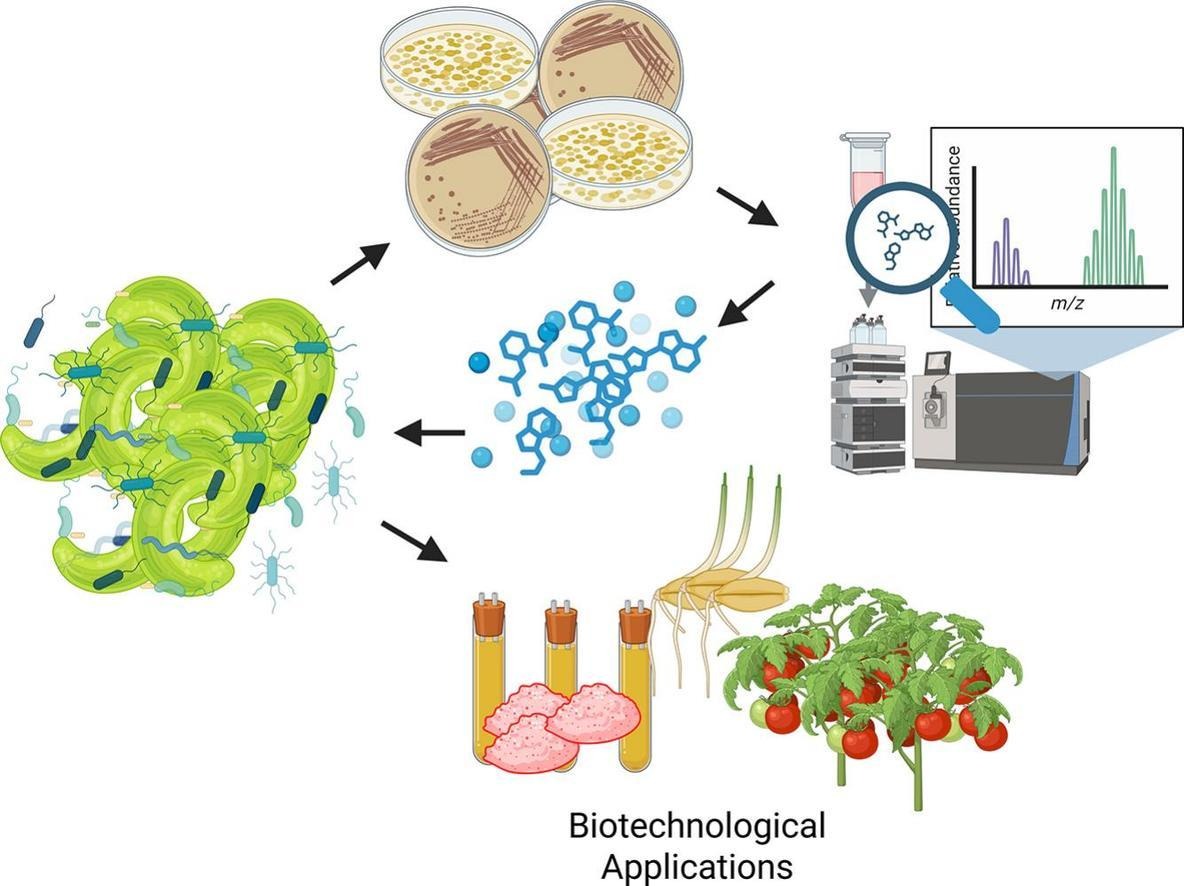
Photosynthetic organisms like microalgae can collect solar energy and transform it into biochemical compounds as other forms of energy that can be utilized in metabolic processes. In nature, microalgae coexist with bacterial communities and may maintain a symbiotic relationship. In the current study, a heterotrophic bacterium, Stenotrophomonas maltophilia was isolated from the phycosphere of a cold-adapted green microalga Monoraphidium sp. (further abbreviated as Monoraphidium). By using advanced liquid chromatography-high-resolution tandem mass spectrometry (LC-HRMS/MS), we were able to detect homoserine lactones (HSLs): 3OHC12-HSL, 3OHC10-HSL, 3OHC14-HSL, C10-HSL, C8-HSL, and OC14-HSL, produced by S. maltophilia. Further, the role of this bacterium in establishing intricate relationships and its implication on biotechnological potential was evaluated. Significant improvements were found in the lutein production of the Monoraphidium culture with bacterial supplements, achieving about 19.3 ± 0.88 mg g−1 DW of this carotenoid compared to 13.7 ± 1.87 mg g−1 DW in the control, which represents an increase of about 40 %. Furthermore, the biostimulant potential of Monoraphidium was evaluated using the germination tests with tomato and barley seeds. A higher germination index was observed with improvements of 55 % in tomato and 110 % in barley, respectively, as compared to the control culture, which was related to the microalgae’s growth stage. The role of the bacterium was evaluated in how the intricate relationships with the microalgal culture can affect its biotechnological potential (e.g., biostimulant activity and lutein production). The current work expands our knowledge towards designing an efficient polyculture based on complementary traits and metabolic potential to maximize the yield and bioactivity in algal biotechnology.



